publications
+: co-first authors
2024
-
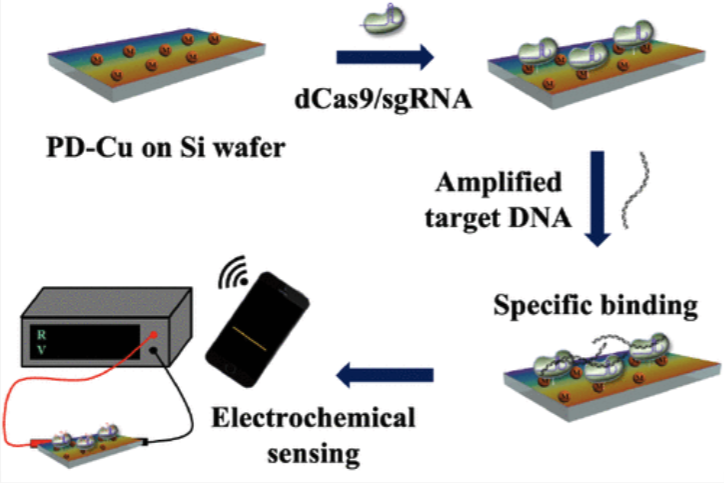 A Wireless, CRISPR-Polymer Dot Electrochemical Sensor for the Diagnosis of Bacterial Pneumonia and Multi-Drug ResistanceSan Hae Im†, Akhmad Irhas Robby†, Heewon Choi, and 4 more authorsACS Applied Materials & Interfaces, 2024PMID: 38278531
A Wireless, CRISPR-Polymer Dot Electrochemical Sensor for the Diagnosis of Bacterial Pneumonia and Multi-Drug ResistanceSan Hae Im†, Akhmad Irhas Robby†, Heewon Choi, and 4 more authorsACS Applied Materials & Interfaces, 2024PMID: 38278531Rapid and accurate diagnosis is crucial for managing the global health threat posed by multidrug-resistant bacterial infections, however, current methods have limitations in either being time-consuming, labor-intensive, or requiring instruments with high costs. Addressing these challenges, we introduce a wireless electrochemical sensor integrating the CRISPR/Cas system with electroconductive polymer dot (PD) nanoparticles to rapidly detect bacterial pathogens from human sputum. To enhance the electroconductive properties, we synthesized copper-ion immobilized PD (PD-Cu), followed by conjugation of the deactivated Cas9 protein (dCas9) onto PD-Cu-coated Si electrodes to generate the dCas9-PD-Cu sensor. The dCas9-PD-Cu sensor integrated with isothermal amplification can specifically detect target nucleic acids of multidrug-resistant bacteria, such as the antibiotic resistance genes kpc-2 and mecA. The dCas9-PD-Cu sensor exhibits high sensitivity, allowing for the detection of 54 femtograms of target nucleic acids, based on measuring the changes in resistivity of the Si electrodes through target capture by dCas9. Furthermore, a wireless sensing platform of the dCas9-PD-Cu sensor was established using a Bluetooth module and microcontroller unit for detection using a smartphone. We demonstrate the feasibility of the platform in diagnosing multidrug-resistant bacterial pneumonia in patients’ sputum samples, achieving 92% accuracy. The current study presents a versatile biosensor platform that can overcome the limitations of conventional diagnostics in the clinic.
-
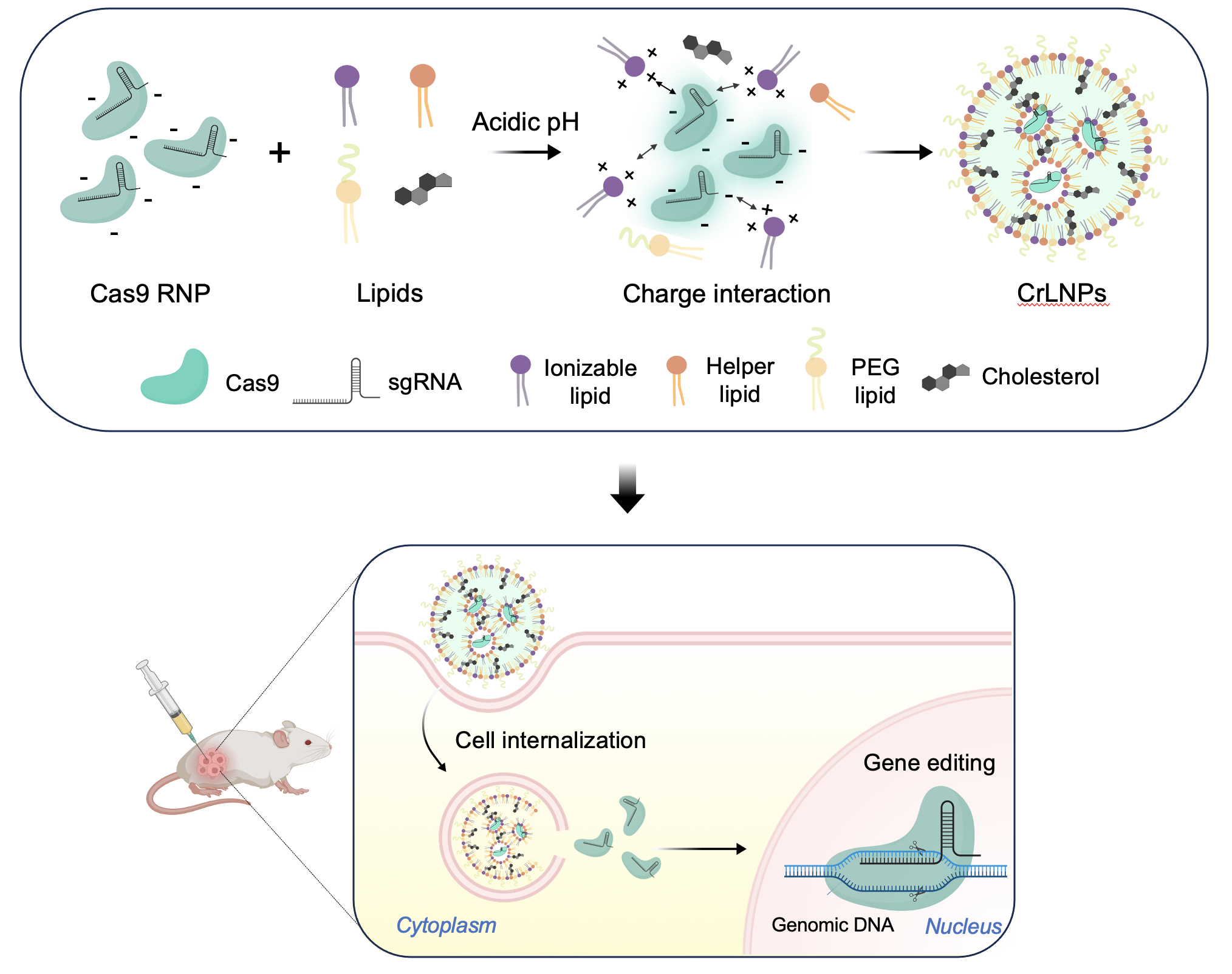 Finely Tuned Ionizable Lipid Nanoparticles for CRISPR/Cas9 Ribonucleoprotein Delivery and Gene EditingSan Hae Im†, Mincheol Jang†, Ji-Ho Park*, and 1 more authorJournal of Nanobiotechnology, 2024
Finely Tuned Ionizable Lipid Nanoparticles for CRISPR/Cas9 Ribonucleoprotein Delivery and Gene EditingSan Hae Im†, Mincheol Jang†, Ji-Ho Park*, and 1 more authorJournal of Nanobiotechnology, 2024Nonviral delivery of the CRISPR/Cas9 system provides great benefits for in vivo gene therapy due to the low risk of side effects. However, in vivo gene editing by delivering the Cas9 ribonucleoprotein (RNP) is challenging due to the poor delivery into target tissues and cells. Here, we introduce an effective delivery method for the CRISPR/Cas9 RNPs by finely tuning the formulation of ionizable lipid nanoparticles. The LNPs delivering CRISPR/Cas9 RNPs (CrLNPs) are demonstrated to induce gene editing with high efficiencies in various cancer cell lines in vitro. Furthermore, we show that CrLNPs can be delivered into tumor tissues with high efficiency, as well as induce significant gene editing in vivo. The current study presents an effective platform for nonviral delivery of the CRISPR/Cas9 system that can be applied as an in vivo gene editing therapeutic for treating various diseases such as cancer and genetic disorders.
-
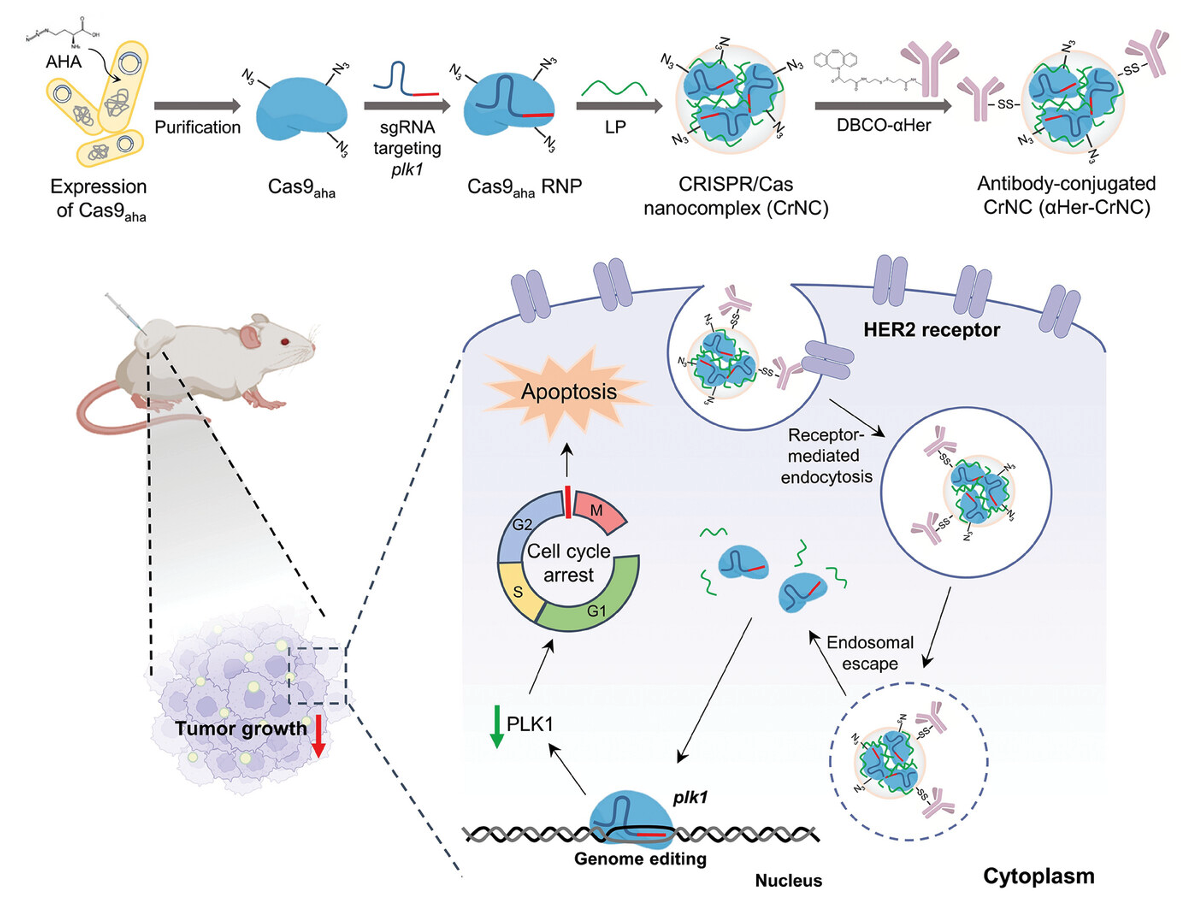 An Antibody-CRISPR/Cas Conjugate Platform for Target-Specific Delivery and Gene Editing in CancerSeungju Yang, San Hae Im, Ju Yeon Chung, and 4 more authorsAdvanced Science, 2024
An Antibody-CRISPR/Cas Conjugate Platform for Target-Specific Delivery and Gene Editing in CancerSeungju Yang, San Hae Im, Ju Yeon Chung, and 4 more authorsAdvanced Science, 2024Abstract The CRISPR/Cas system has been introduced as an innovative tool for therapy, however achieving specific delivery to the target has been a major challenge. Here, an antibody-CRISPR/Cas conjugate platform that enables specific delivery and target gene editing in HER2-positive cancer is introduced. The CRISPR/Cas system by replacing specific residues of Cas9 with an unnatural amino acid is engineered, that can be complexed with a nanocarrier and bioorthogonally functionalized with a monoclonal antibody targeting HER2. The resultant antibody-conjugated CRISPR/Cas nanocomplexes can be specifically delivered and induce gene editing in HER2-positive cancer cells in vitro. It is demonstrated that the in vivo delivery of the antibody-CRISPR/Cas nanocomplexes can effectively disrupt the plk1 gene in HER2-positive ovarian cancer, resulting in substantial suppression of tumor growth. The current study presents a useful therapeutic platform for antibody-mediated delivery of CRISPR/Cas for the treatment of various cancers and genetic diseases.
2023
-
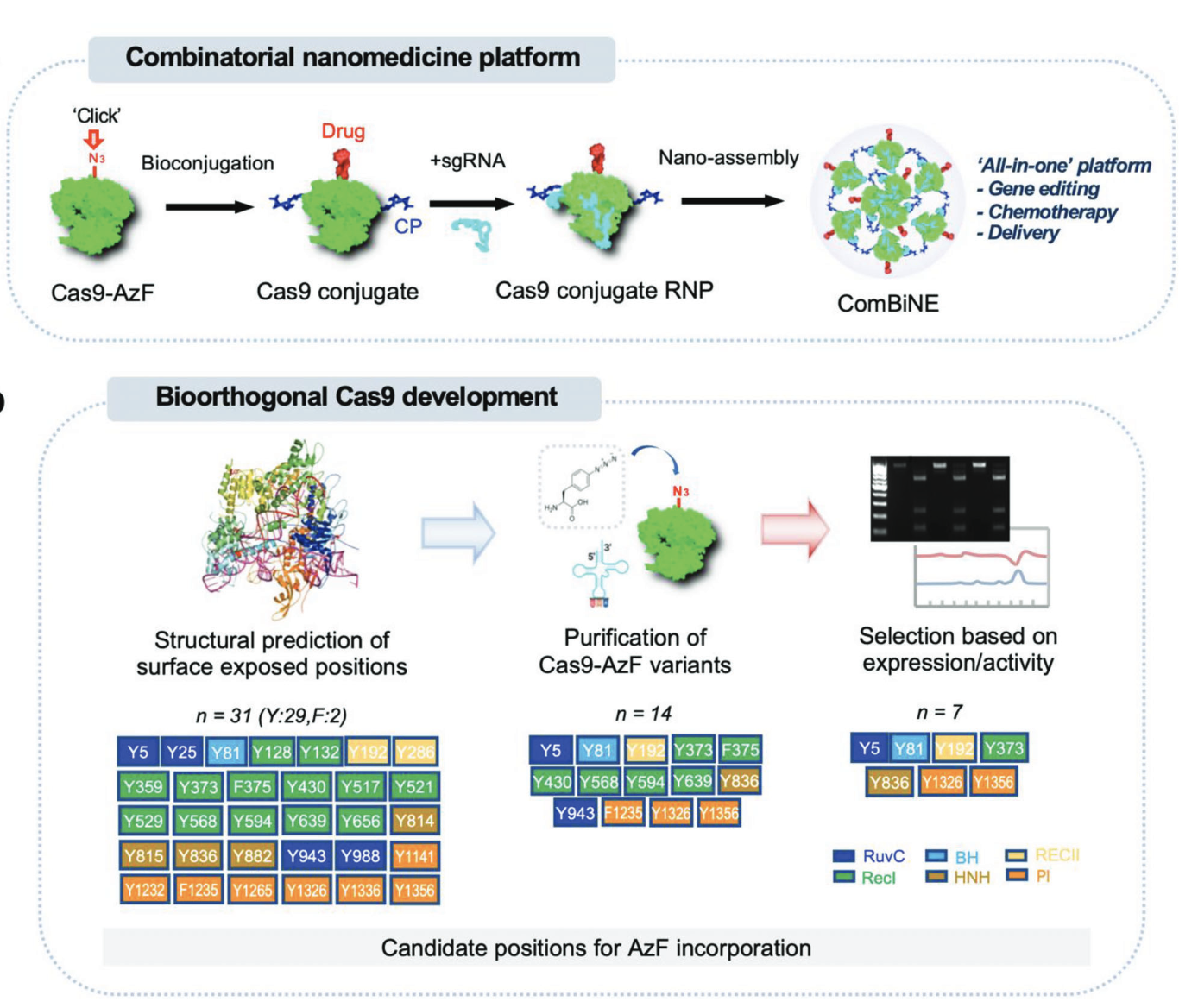 Bioorthogonal CRISPR/Cas9-Drug Conjugate: A Combinatorial Nanomedicine PlatformMarcel Janis Beha†, Joo-Chan Kim†, San Hae Im†, and 7 more authorsAdvanced Science, 2023
Bioorthogonal CRISPR/Cas9-Drug Conjugate: A Combinatorial Nanomedicine PlatformMarcel Janis Beha†, Joo-Chan Kim†, San Hae Im†, and 7 more authorsAdvanced Science, 2023Bioconjugation of proteins can substantially expand the opportunities in biopharmaceutical development, however, applications are limited for the gene editing machinery despite its tremendous therapeutic potential. Here, a self-delivered nanomedicine platform based on bioorthogonal CRISPR/Cas9 conjugates, which can be armed with a chemotherapeutic drug for combinatorial therapy is introduced. It is demonstrated that multi-functionalized Cas9 with a drug and polymer can form self-condensed nanocomplexes, and induce significant gene editing upon delivery while avoiding the use of a conventional carrier formulation. It is shown that the nanomedicine platform can be applied for combinatorial therapy by incorporating the anti-cancer drug olaparib and targeting the RAD52 gene, leading to significant anti-tumor effects in BRCA-mutant cancer. The current development provides a versatile nanomedicine platform for combination treatment of human diseases such as cancer.
-
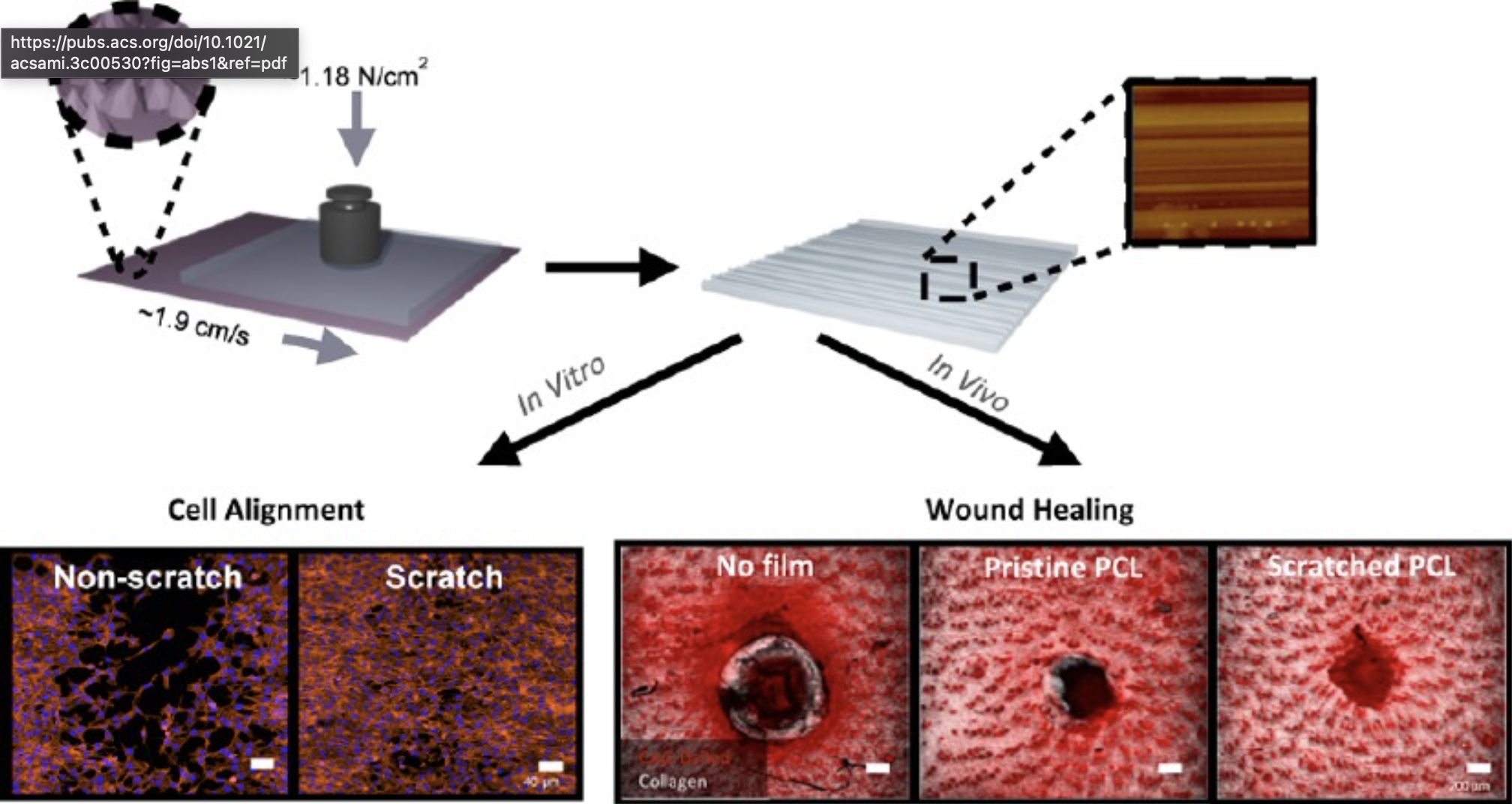 Fabrication of Scratched Nanogrooves for Highly Oriented Cell Alignment and Application as a Wound Healing DressingMin Jeong Shin†, San Hae Im†, Baekman Kim†, and 7 more authorsACS Applied Materials & Interfaces, 2023PMID: 37014981
Fabrication of Scratched Nanogrooves for Highly Oriented Cell Alignment and Application as a Wound Healing DressingMin Jeong Shin†, San Hae Im†, Baekman Kim†, and 7 more authorsACS Applied Materials & Interfaces, 2023PMID: 37014981Using improper wound care materials may cause impaired wound healing, which can involve scar formation and infection. Herein, we propose a facile method to fabricate a cell-alignment scaffold, which can effectively enhance cell growth and migration, leading to the reproduction of cellular arrangements and restoration of tissues. The principle is scratching a diamond lapping film that gives uniaxial nanotopography on substrates. Cells are seeded to follow the geometric cue via contact guidance, resulting in highly oriented cell alignment. Remarkable biocompatibility is also demonstrated by the high cell viability on various substrates. In vivo studies in a wound healing model in mice show that the scratched film supports directed cell guidance on the nanostructure, with significantly reduced wound areas and inhibition of excessive collagen deposition. Rapid recovery of the epidermis and dermis is also shown by histological analyses, suggesting the potential application of the scratching technique as an advanced wound dressing material for effective tissue regeneration.
-
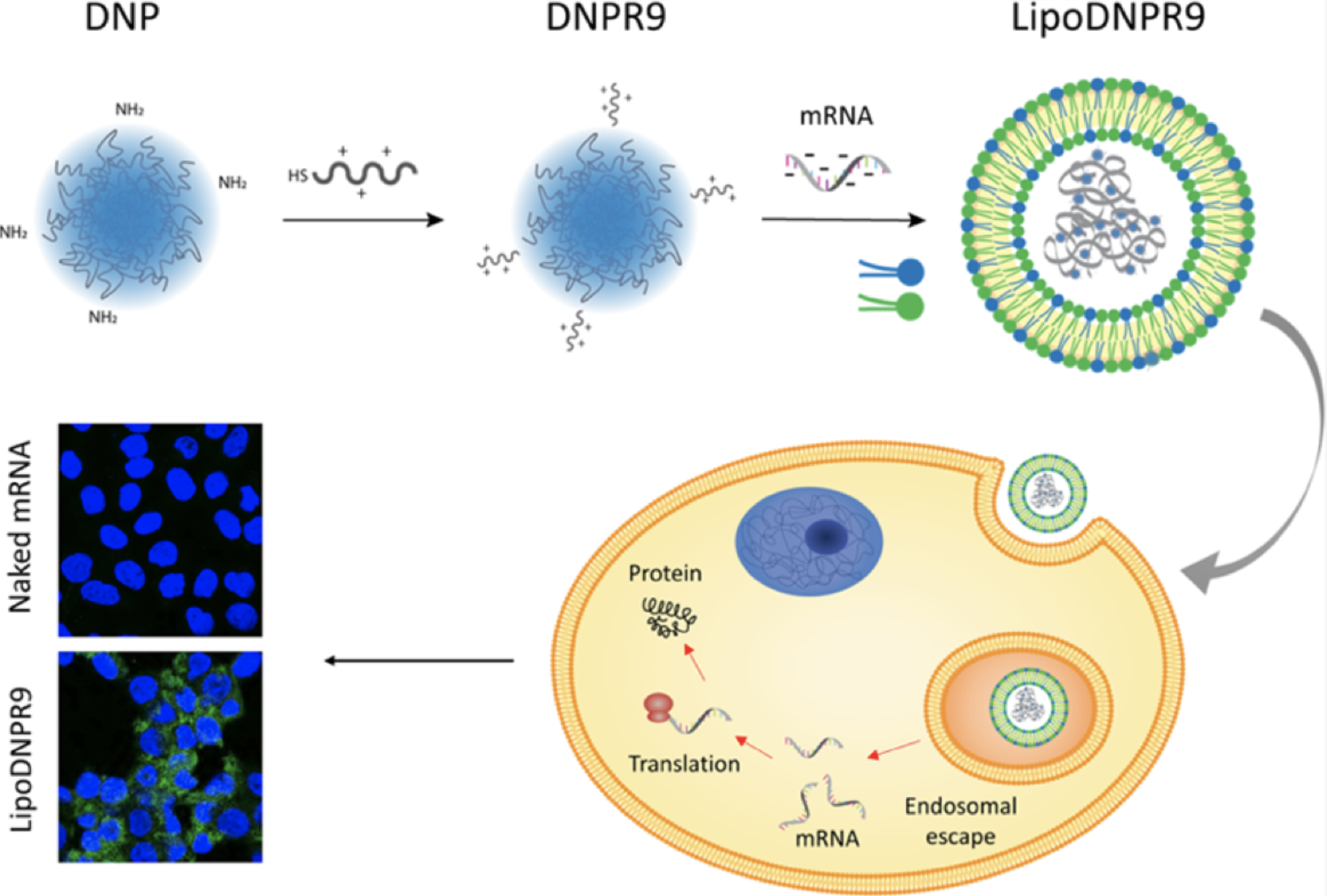 Effective mRNA Delivery by Condensation with Cationic Nanogels Incorporated into LiposomesNevena Duskunovic, San Hae Im, Juhee Lee, and 1 more authorMolecular Pharmaceutics, 2023PMID: 37184833
Effective mRNA Delivery by Condensation with Cationic Nanogels Incorporated into LiposomesNevena Duskunovic, San Hae Im, Juhee Lee, and 1 more authorMolecular Pharmaceutics, 2023PMID: 37184833The challenge in effective delivery of mRNA has been a major hurdle in their development as therapeutics. Herein, we present that the incorporation of cationic nanogels as the condensing material for mRNA into liposomes enables stable and enhanced mRNA delivery to cells in vitro. We prepared dextran-based nanogel particles, which were surface functionalized with oligoarginine peptide (DNPR9) and complexed with mRNA for incorporation into liposomes (LipoDNPR9). The use of DNPR9 with the liposomes resulted in enhanced internalization, as well as a 4-fold increase in transfection of luciferase mRNA when treated with A549 cells in vitro, compared to control liposomes. The enhancement in transfection efficiency was also observed in various cell lines while causing low cytotoxicity. The versatility of the strategy was also investigated by applying DNPR9 for mRNA condensation to ionizable lipid particles, which resulted in an ∼55% increase in transfection. The current development based on nanogel-incorporated liposomes introduces an effective platform for mRNA delivery, while the condensation strategy using DNPR9 can be widely applied for various lipid-based formulations to enhance their efficacy.
2022
-
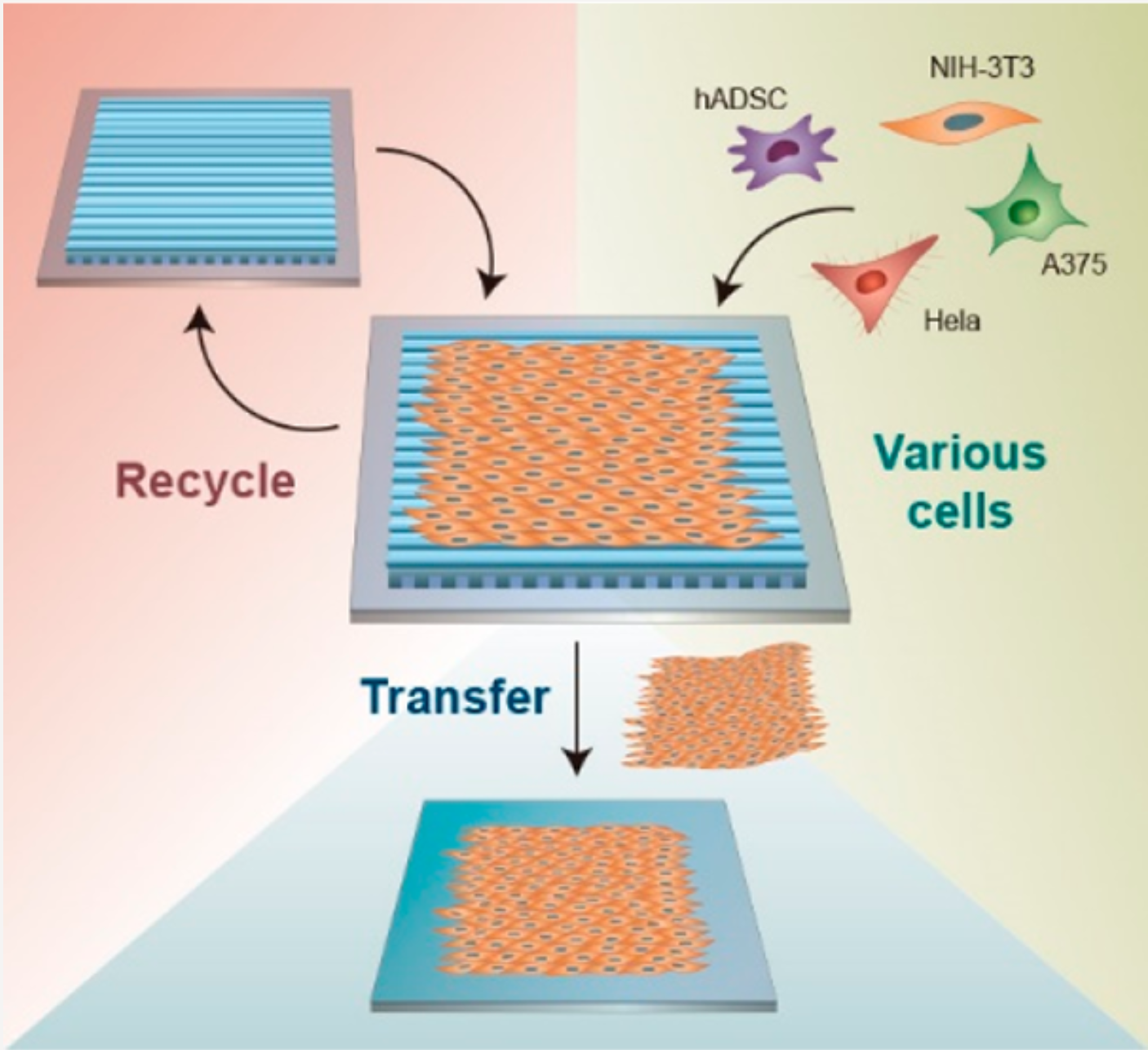 Recyclable Periodic Nanostructure Formed by Sublimable Liquid Crystals for Robust Cell AlignmentMin Jeong Shin†, San Hae Im†, Wantae Kim, and 4 more authorsLangmuir, 2022PMID: 35302783
Recyclable Periodic Nanostructure Formed by Sublimable Liquid Crystals for Robust Cell AlignmentMin Jeong Shin†, San Hae Im†, Wantae Kim, and 4 more authorsLangmuir, 2022PMID: 35302783We demonstrate a facile method to fabricate a recyclable cell-alignment scaffold using nanogrooves based on sublimable liquid crystal (LC) material. Randomly and uniaxially arranged smectic LC structures are obtained, followed by sublimation and recondensation processes, which directly produce periodic nanogrooves with dimensions of a couple of hundreds of nanometers. After treatment with osmium tetroxide (OsO4), the nanogroove can serve as a scaffold to efficiently induce directed cell growth without causing cytotoxicity, and it can be used repeatedly. Together, various cell types are applied to the nanogroove, proving the scaffold’s broad applicability. Depending on the nanotopography of the LC structures, cells exhibit different morphologies and gene expression patterns, compared to cells on standard glass substrates, according to microscopic observation and qPCR. Furthermore, cell sheets can be formed, which consist of oriented cells that can be repeatedly formed and transferred to other substrates, while maintaining its organization. We believe that our cell-aligning scaffold may pave the way for the soft material field to bioengineering, which can involve fundamentals in cell behavior and function, as well as applications for regenerative medicine.
-
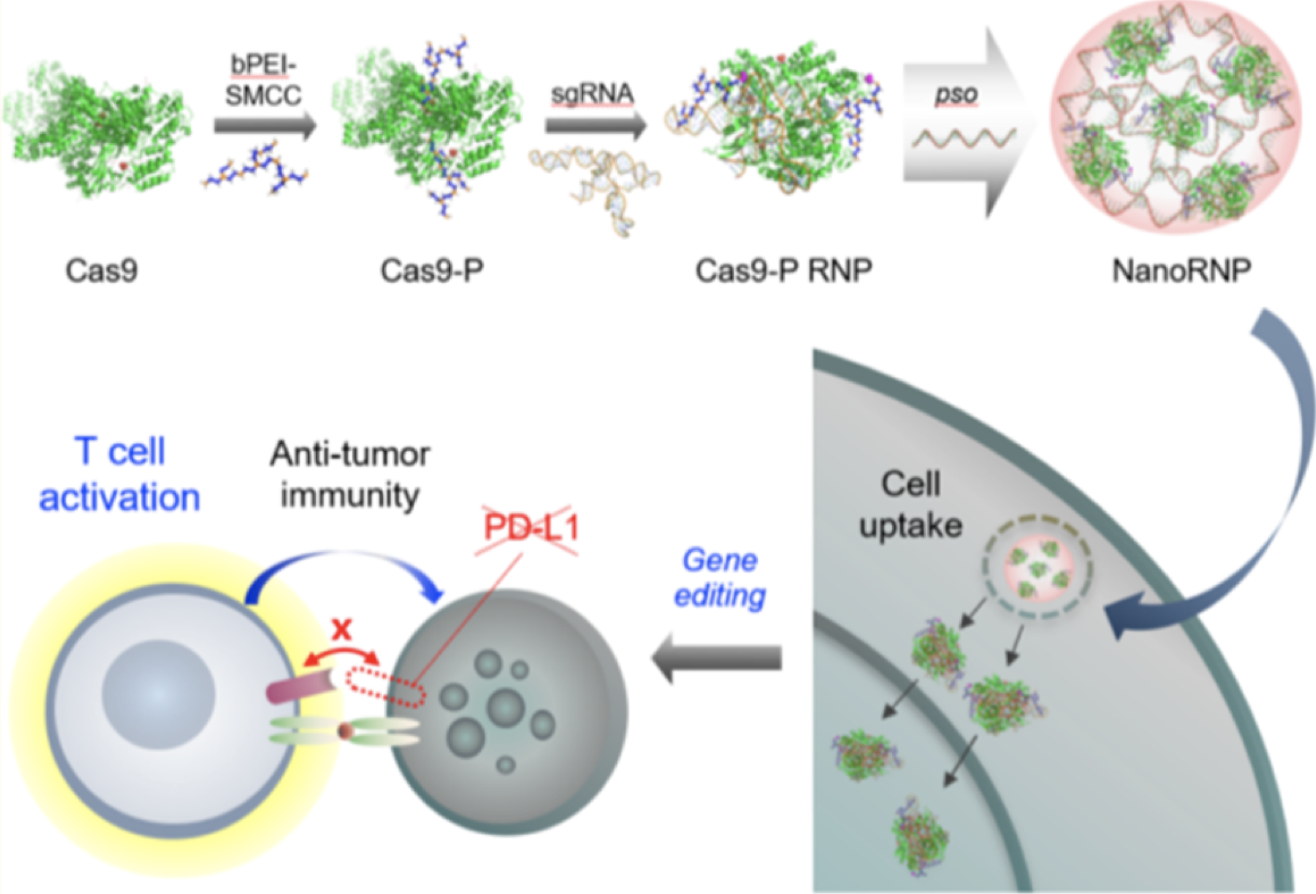 Nano-assembly of a Chemically Tailored Cas9 Ribonucleoprotein for In Vivo Gene Editing and Cancer ImmunotherapyJuhee Lee†, Yoo Kyung Kang†, Eonju Oh, and 7 more authorsChemistry of Materials, 2022
Nano-assembly of a Chemically Tailored Cas9 Ribonucleoprotein for In Vivo Gene Editing and Cancer ImmunotherapyJuhee Lee†, Yoo Kyung Kang†, Eonju Oh, and 7 more authorsChemistry of Materials, 2022Cancer gene therapy based on the clustered regularly interspaced short palindromic repeat (CRISPR) system has been challenging due to the poor delivery and efficacy in vivo. Herein, we report the development of nanoassembled ribonucleoprotein complexes (NanoRNP), which can efficiently block the PD-L1 immune checkpoint and induce an anti-tumor effect in vivo. We utilize CRISPR-associated protein 9 (Cas9) that is chemically derivatized with a low-molecular weight polymer, which condenses with single-guide RNA and modified DNA oligonucleotides to form stabilized NanoRNPs. Delivery of the NanoRNP into B16 melanoma cells in vitro leads to efficient internalization and gene disruption with low cytotoxicity, leading to sustained downregulation of PD-L1. In vivo delivery in a mouse melanoma model demonstrates that the NanoRNP can induce indels in the target cells of the tumor at high frequencies, resulting in major suppression of tumor growth, without involving combinatorial treatment. Blockade of the PD-L1 checkpoint by the NanoRNP in tumor tissues promotes T cell infiltration and effector cytokine release, which are characteristics of the activation of anti-tumor immunity, and inhibition of immunosuppressive myeloid cells. The current system suggests a promising strategy as an in vivo gene editing platform for cancer immunotherapy.
2021
-
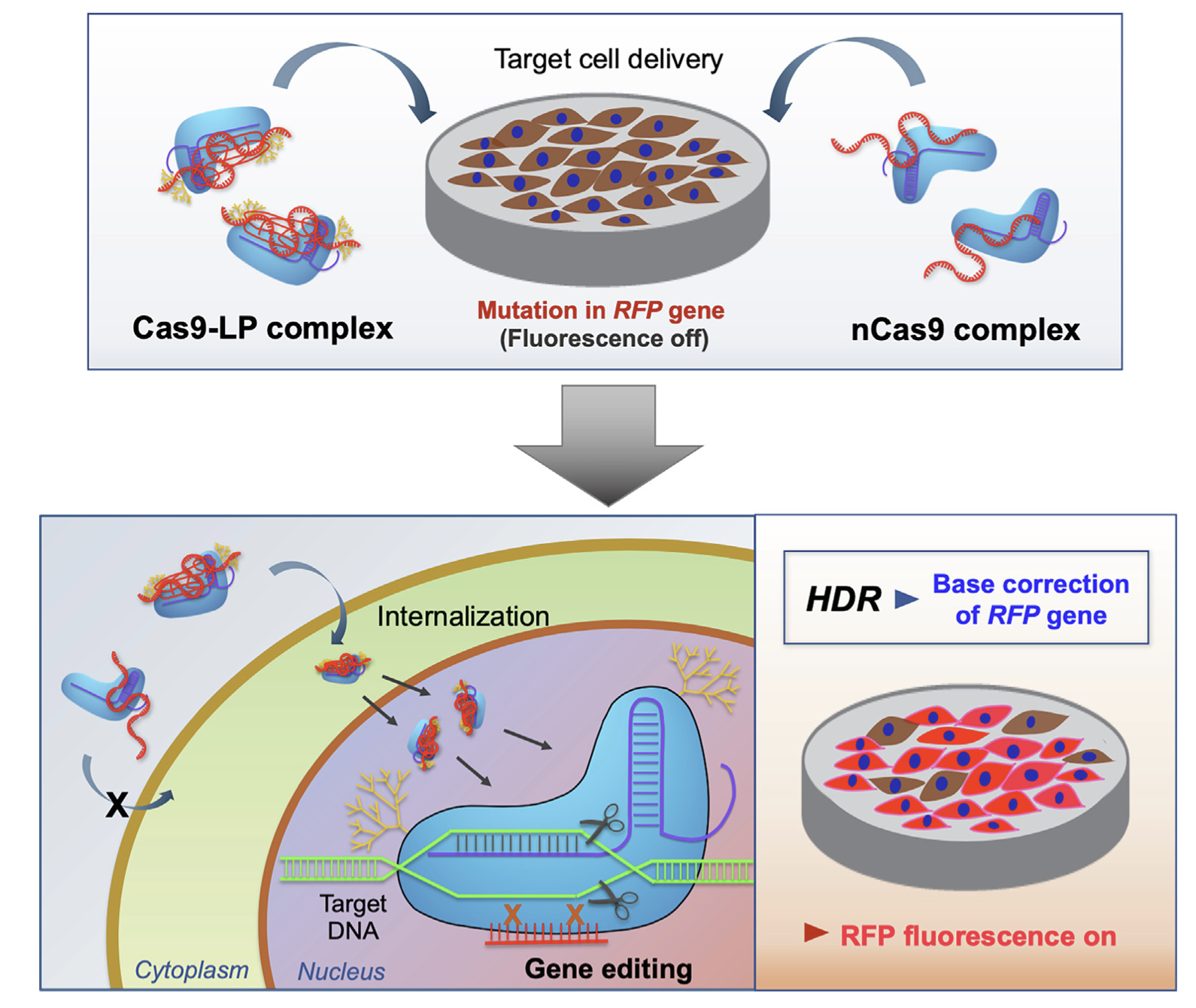 Cas9 conjugate complex delivering donor DNA for efficient gene editing by homology-directed repairYoo Kyung Kang, Juhee Lee, San Hae Im, and 7 more authorsJournal of Industrial and Engineering Chemistry, 2021
Cas9 conjugate complex delivering donor DNA for efficient gene editing by homology-directed repairYoo Kyung Kang, Juhee Lee, San Hae Im, and 7 more authorsJournal of Industrial and Engineering Chemistry, 2021Delivery of the CRISPR ribonucleoprotein (RNP) for homology-directed repair (HDR) has been challenging due to the low efficiency. Herein, we developed a Cas9 conjugate complex system which can induce efficient HDR editing with the use of a minimal amount of carrier material. Cas9 from Streptococcus pyogenes was purified and conjugated with low molecular weight polymer (LP). The Cas9-LP conjugates were complexed with single guide RNA (sgRNA) and donor DNA, which showed greatly enhanced internalization into cells compared to native Cas9 complexes, as well as a high extent of co-localization of Cas9 with sgRNA. The cytotoxicity of Cas9-LP complexes was evaluated, demonstrating low cytotoxicity compared to the conventional lipofectamine formulation. Finally, the treatment of Cas9-LP complexes to HEK293T reporter cell line expressing a mutant red fluorescent protein (RFP) results in efficient base correction of the RFP gene (up to 31%), leading to restoration of RFP expression and fluorescence. We anticipate that the current method can be widely used as a platform for efficient HDR editing via ‘minimal carrier-assisted’ delivery without the aid of any external physical stimuli, which can be potentially applied for in vivo and ex vivo editing of cellular targets.
2020
-
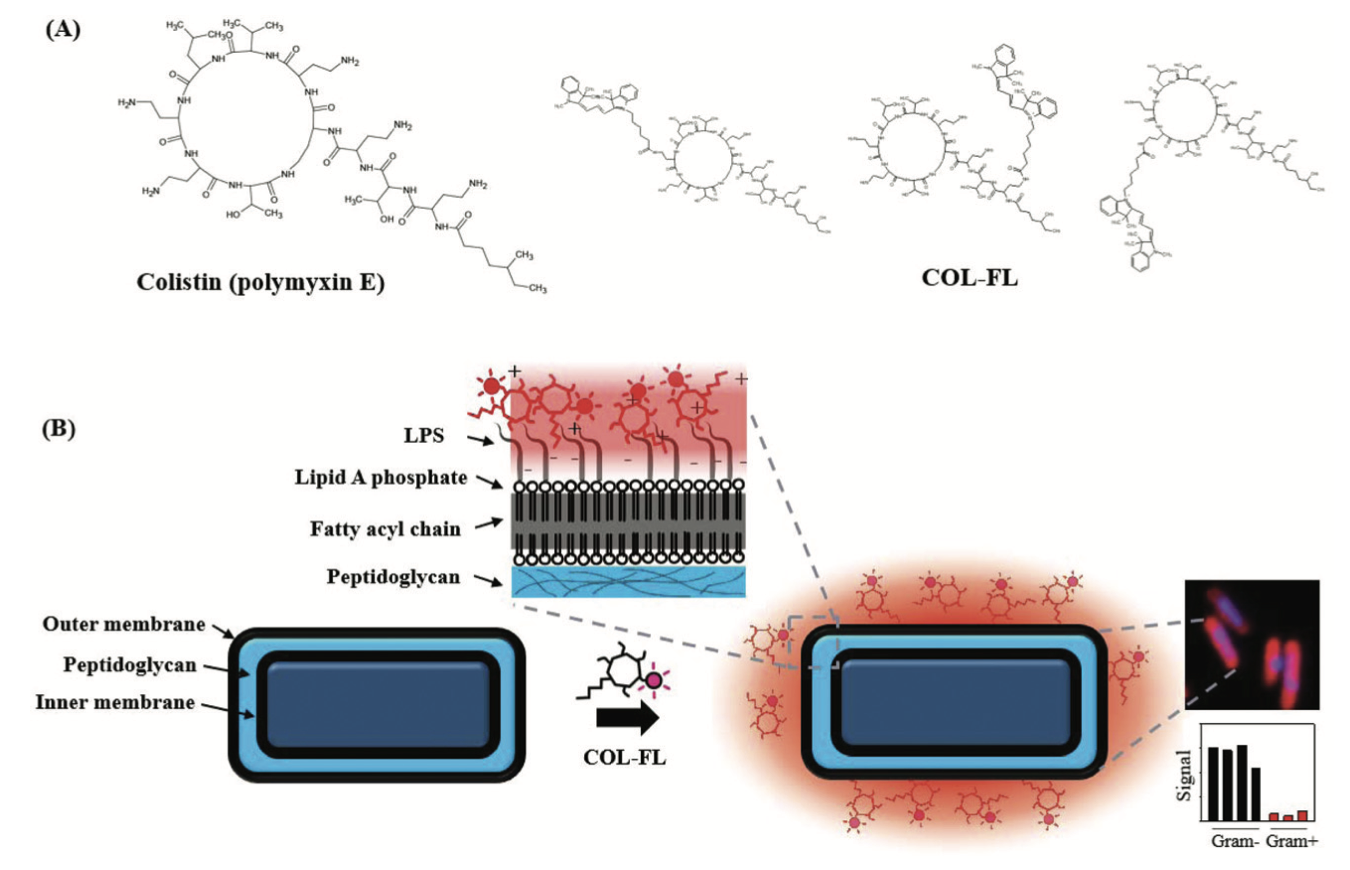 Ultra-fast and universal detection of Gram-negative bacteria in complex samples based on colistin derivativesJea Sung Ryu, San Hae Im, Yoo Kyung Kang, and 2 more authorsBiomater. Sci., 2020
Ultra-fast and universal detection of Gram-negative bacteria in complex samples based on colistin derivativesJea Sung Ryu, San Hae Im, Yoo Kyung Kang, and 2 more authorsBiomater. Sci., 2020Gram-negative bacteria are a significant cause of infections acquired in both hospital and community settings, resulting in a high mortality rate worldwide. Currently, a Gram-negative infection is diagnosed by symptom evaluation and is treated with empiric antibiotics which target both Gram-negative and Gram-positive bacteria. A rapid and simple diagnostic method would enable immediate and targeted treatment, while dramatically reducing antibiotic overuse. Herein, we introduce a method utilizing a fluorescent derivative of colistin (COL-FL), that can directly label the Gram-negative cell wall of live bacteria and universally detect the targets within 10 min. By using the COL-FL assay, we achieved the differential labeling of various Gram-negative pathogens related to hospital-acquired infections, which could be subsequently detected via spectrofluorometry and microscopy. Further, we determined that our method can be used for complex samples, such as combinations of multiple bacterial types; bacteria in the presence of mammalian cells; and bacteria with serum components. This assay can be integrated into a simple diagnostic platform for rapid screening tests and the stratification of Gram-negative bacterial infections in the clinic.
-
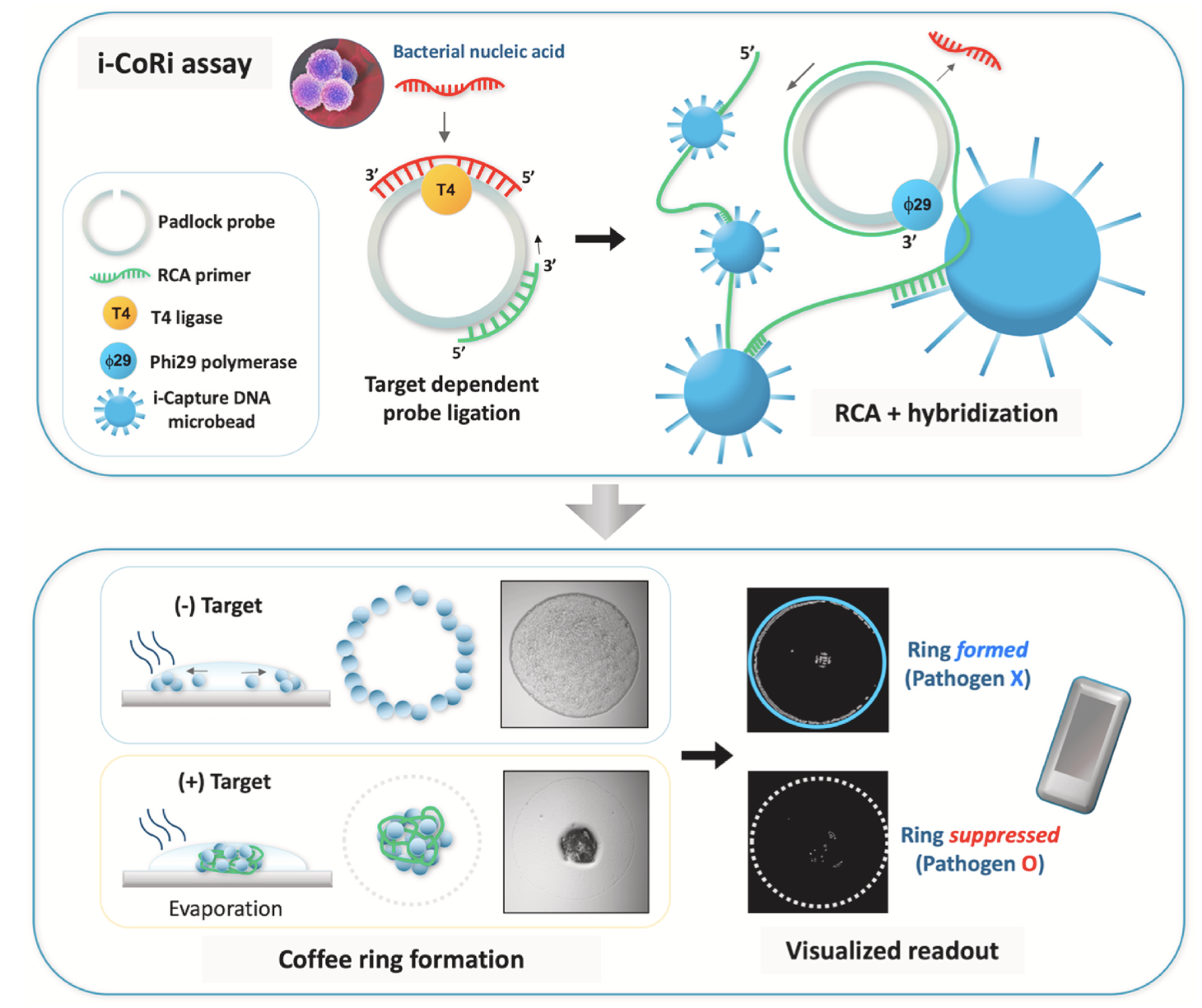 Simple visualized readout of suppressed coffee ring patterns for rapid and isothermal genetic testing of antibacterial resistanceYoo Kyung Kang, San Hae Im, Jea Sung Ryu, and 2 more authorsBiosensors and Bioelectronics, 2020
Simple visualized readout of suppressed coffee ring patterns for rapid and isothermal genetic testing of antibacterial resistanceYoo Kyung Kang, San Hae Im, Jea Sung Ryu, and 2 more authorsBiosensors and Bioelectronics, 2020We present a facile method based on the coffee ring effect that can rapidly detect antibiotic-resistant bacteria, as an affordable genetic testing platform. When a colloidal solution of particles is dropped onto a substrate surface, an outward capillary flow upon evaporation induces the migration of the particles to the periphery of the droplet, forming a characteristic ring pattern. Herein, we utilize capture DNA microbeads which in the presence of target nucleic acid, form suppressed ring patterns by hybridization-induced crosslinking of the microbeads. The coffee ring-based assay is integrated with isothermal amplification based on rolling circle amplification (RCA), to produce long, single-stranded target DNA and induce hybridization, via a one-step procedure (i-CoRi assay). The resultant ring patterns can be simply observed with the naked eye or recorded with a standard mobile device for readout. The i-CoRi assay was validated for the rapid and specific detection of the antibiotic resistance gene mecA for MRSA, showing that detection was possible at the sub-zeptomolar range ( 0.2zM) with the specificity of distinguishing 2 mismatched bases. The spatial patterns of the microbeads were characterized, showing the dense packing of the microbeads at the center of the droplet and thinning of the ring pattern for the MRSA target, which were distinct from the negative controls MSSA, E. coli, and P. aeruginosa. The images of the microbead patterns were also processed by a simple readout algorithm to discriminate the presence or absence of the coffee ring, to enable diagnostic decision making. The current method provides a rapid and versatile platform for the specific identification of bacterial pathogens and multidrug resistance, especially for diagnosis in resource-limited settings.